When treating hormone receptor‑positive breast cancer, Ribociclib is a selective cyclin‑dependent kinase 4/6 (CDK4/6) inhibitor approved to slow tumor growth by blocking cell‑cycle progression. It’s part of a newer wave of targeted therapies that give patients a real chance at longer, healthier lives.
What is Ribociclib?
Ribociclib (brand name KISQALI) hit the U.S. market in 2017 after the FDA green‑lighted it for use with an aromatase inhibitor in post‑menopausal women whose tumors are hormone‑driven. Chemically, it locks CDK4 and CDK6 in an inactive state, preventing the phosphorylation of the retinoblastoma protein-a key step that normally tells a cell to divide.
How does it work in hormone receptor‑positive breast cancer?
The disease we’re talking about is Hormone Receptor‑Positive Breast Cancer, which means the tumor cells grow when they sense estrogen or progesterone. By pairing ribociclib with an aromatase inhibitor (which slashes estrogen production), you essentially cut off both the fuel and the ignition. The result is a double‑hit that keeps the cancer from proliferating.
Clinical evidence - key trials
The backbone of ribociclib’s reputation comes from the MONALEESA series of phase III trials. In MONALEESA‑2, 666 women received ribociclib + letrozole versus placebo + letrozole. Median progression‑free survival (PFS) jumped from 14.7 months to 25.3 months-a 70% improvement. MONALEESA‑7 focused on pre‑menopausal women (who need ovarian suppression) and showed a median overall survival gain of 9.4 months. MONALEESA‑3 added fulvestrant as the partner drug and still delivered a PFS benefit of 20.5 months versus 12.8 months.
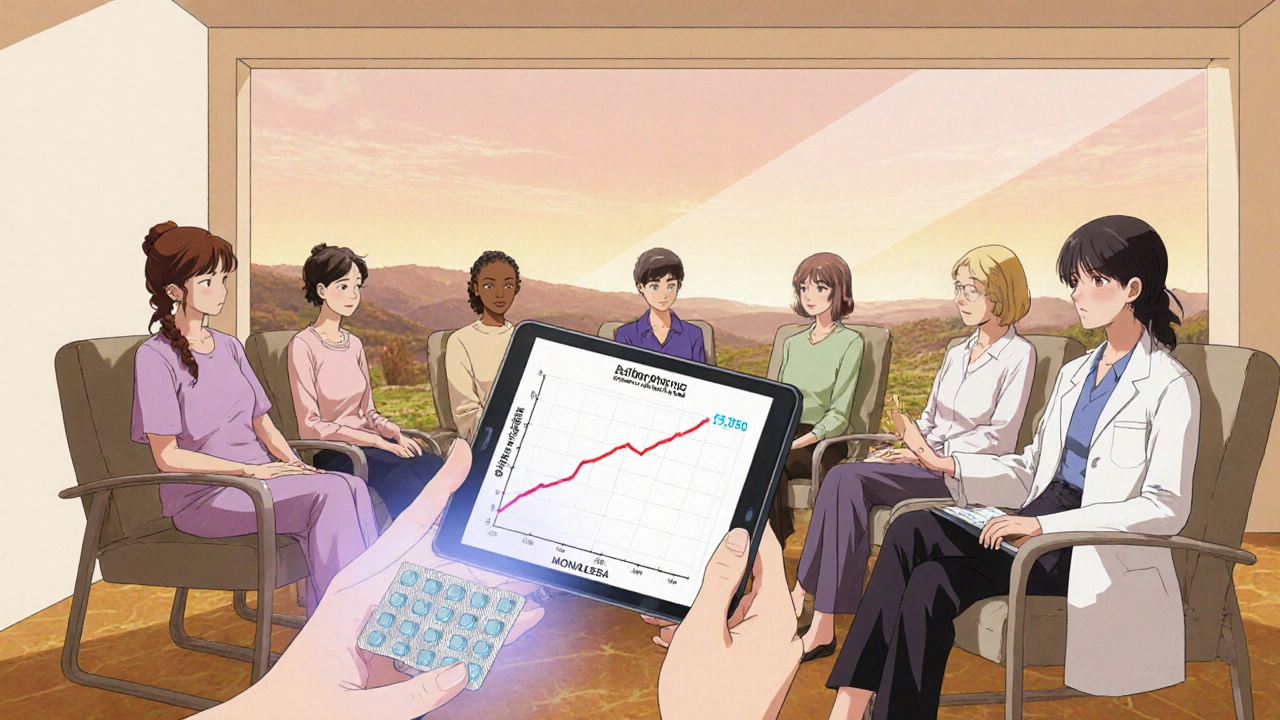
Approved dosing and administration
- Standard regimen: 600 mg (three 200 mg tablets) taken orally once daily for 21 days, followed by a 7‑day break (3‑week‑on/1‑week‑off cycle).
- Take with food; avoid grapefruit juice because it can boost drug levels.
- Routine blood work is required before starting and every 2 weeks for the first two cycles, then monthly, to monitor liver enzymes and neutrophil counts.
- If liver enzymes climb > 2.5 × ULN or neutrophils drop < 1,000/µL, dose reduction or interruption is recommended.
Side effects you should watch for
Like any cancer drug, ribociclib isn’t free of drawbacks. The most common adverse events (≥ 20% of patients) include:
- Neutropenia - low white‑blood‑cell count, which can increase infection risk.
- Elevated liver enzymes (ALT/AST) - usually reversible with dose adjustment.
- Nausea, fatigue, and alopecia (hair thinning).
- QT‑interval prolongation on ECG - a heart rhythm change that warrants baseline and periodic EKGs, especially for patients on other QT‑prolonging meds.
Most side effects are manageable with dose modifications or supportive care. Promptly reporting fevers, chills, or yellowing of the skin/eyes can prevent serious complications.
How does Ribociclib compare with other CDK4/6 inhibitors?
| Attribute | Ribociclib (KISQALI) | Palbociclib (Ibrance) | Abemaciclib (Verzenio) |
|---|---|---|---|
| FDA approval year (HR+ BC) | 2017 | 2015 | 2018 |
| Typical dosing schedule | 600 mg 3 weeks on / 1 week off | 125 mg 3 weeks on / 1 week off | 150 mg continuously (twice daily) |
| Most common grade 3/4 adverse event | Neutropenia (≈ 60%) | Neutropenia (≈ 65%) | Diarrhea (≈ 20%) |
| PFS benefit in pivotal trial (months) | + 10.6 (MONALEESA‑2) | + 9.5 (PALOMA‑2) | + 8.3 (MONARCH‑2) |
| QT‑interval effect | Yes (monitor) | None noted | None noted |
All three drugs share the same basic mechanism-blocking CDK4/6-but they differ in dosing convenience, side‑effect profile, and subtle efficacy nuances. If you have a history of heart rhythm issues, ribociclib’s QT‑prolongation risk may push you toward palbociclib or abemaciclib. Conversely, if chronic diarrhea is a concern, ribociclib or palbociclib might be better choices.

Practical checklist for patients starting Ribociclib
- Confirm tumor is hormone‑receptor‑positive and HER2‑negative.
- Discuss baseline liver function tests and ECG with your oncologist.
- Arrange a regular schedule for blood work (every 2 weeks → then monthly).
- Set up a reminder for the 21‑day‑on/7‑day‑off pill schedule.
- Keep a symptom diary (fever, fatigue, nausea, heart palpitations).
- Know the dose‑reduction steps: 600 mg → 400 mg → 200 mg if labs become abnormal.
- Ask about patient‑assistance programs; many insurers cover ribociclib but pre‑auth may be needed.
Insurance, access, and cost considerations
The FDA approved ribociclib for the indicated setting, which usually guarantees coverage under most private plans and Medicare Part B (with an oral oncology benefit). However, the drug’s list price hovers around $12,000 USD per month, so a prior‑authorization request is standard.
Pharmacy‑based patient‑support programs-often run by the manufacturer-can provide co‑pay assistance or even free medication for qualifying patients. Ask your oncology clinic’s financial counselor to submit the paperwork early to avoid treatment delays.
Frequently Asked Questions
Can I take ribociclib if I’m pre‑menopausal?
Yes. In the MONALEESA‑7 trial, pre‑menopausal women received ribociclib together with ovarian suppression (goserelin or leuprolide) and an aromatase inhibitor, showing a clear overall‑survival benefit.
Do I need to stop other meds before starting ribociclib?
Your doctor will review all concurrent drugs. Strong CYP3A4 inhibitors (like ketoconazole) and inducers (like rifampin) can alter ribociclib levels, so dose adjustments or alternatives may be required.
What should I do if my blood tests show high liver enzymes?
Mild elevations (≤ 2.5 × ULN) often resolve on their own. If they rise above that, your oncologist will likely pause ribociclib and re‑check labs in 1‑2 weeks, then restart at a lower dose once they normalize.
Is ribociclib curative?
It isn’t a cure, but it converts many advanced‑stage cancers into a chronic, manageable condition, extending life by years for many patients.
How does ribociclib differ from traditional chemotherapy?
Chemotherapy attacks rapidly dividing cells indiscriminately, causing hair loss, nausea, and low blood counts across the board. Ribociclib targets a specific cell‑cycle checkpoint in hormone‑driven tumors, so side effects are generally milder and more predictable.
Bottom line: ribociclib is a well‑studied, FDA‑approved option that adds a solid survival advantage for patients with hormone receptor‑positive breast cancer. By understanding its mechanism, monitoring requirements, and how it stacks up against its peers, you can make an informed choice and work with your care team to keep the disease in check.

14 Comments
Leo Chan
19 October, 2025Just wanted to say that ribociclib really feels like a game‑changer for many patients. The way it pairs with an aromatase inhibitor gives you that double‑hit you want without the brutal side‑effects of classic chemo. Keeping an eye on blood work and the QT interval makes the regimen manageable, even for folks juggling a busy life. If you’re starting it, set up a pill‑tracker reminder so you don’t miss the 21‑day‑on schedule.
jagdish soni
26 October, 2025One might argue that the hype surrounding CDK4/6 inhibitors borders on reverence, yet the data does not fully absolve us from scrutiny. The MONALEESA trials, while impressive, still leave gaps concerning long‑term cardiac outcomes, especially in patients with pre‑existing QT concerns. Moreover, the cost factor inevitably filters accessibility, rendering the “miracle” narrative somewhat myopic. Nonetheless, the mechanistic elegance cannot be denied.
Latasha Becker
1 November, 2025From a pharmacodynamic standpoint, ribociclib’s selective inhibition of CDK4/6 disrupts the G1‑to‑S phase transition, a hallmark of hormone‑driven oncogenesis.
Unlike non‑selective cytotoxics, this targeted approach spares proliferative compartments such as the gastrointestinal epithelium, curbing the incidence of severe diarrhea seen with agents like abemaciclib.
The pivotal MONALEESA‑2 trial demonstrated a median progression‑free survival of 25.3 months versus 14.7 months with letrozole alone, translating to a hazard ratio of 0.55, which is statistically robust.
In the pre‑menopausal cohort of MONALEESA‑7, the addition of ovarian suppression amplified overall survival by over nine months, underscoring the importance of endocrine context.
Pharmacokinetic interactions remain a critical consideration: strong CYP3A4 inhibitors elevate plasma concentrations, potentially exacerbating neutropenia and hepatic toxicity, while inducers may diminish efficacy.
Regular monitoring of neutrophil counts is mandated bi‑weekly initially, reflecting the drug’s propensity to induce grade 3/4 neutropenia in roughly 60% of patients.
Hepatic enzyme elevations, though usually reversible, require dose adjustments if surpassing 2.5× ULN, aligning with FDA labeling.
The QT‑interval prolongation risk, albeit modest, necessitates baseline and periodic ECGs, particularly when co‑administered with other QT‑affecting agents.
Real‑world evidence suggests adherence rates hover around 80%, with the 21‑days‑on/7‑days‑off schedule facilitating patient compliance compared to continuous dosing regimens.
Cost‑effectiveness analyses remain mixed; while the drug’s list price exceeds $12,000 per month, patient assistance programs mitigate out‑of‑pocket burden for many insured individuals.
Comparative data indicate that ribociclib offers a slightly superior PFS benefit relative to palbociclib, though the latter boasts a more favorable cardiac safety profile.
The choice between CDK4/6 inhibitors should thus be individualized, balancing efficacy, side‑effect tolerability, comorbidities, and socioeconomic factors.
Emerging biomarkers, such as cyclin D1 amplification, may eventually refine patient selection, but current clinical practice relies on hormone‑receptor status and performance status alone.
Importantly, ribociclib does not confer a cure; it transforms metastatic disease into a chronic condition, extending survivorship while preserving quality of life.
Ongoing phase IV trials are evaluating combination strategies with PI3K inhibitors, potentially broadening the therapeutic arsenal in HR+ breast cancer.
parth gajjar
8 November, 2025Whatever the hype, the pill still feels like a decent daily routine.
Maridel Frey
14 November, 2025Thank you for the comprehensive overview; the detailed breakdown of monitoring protocols and pharmacologic interactions will be invaluable for clinicians guiding patients through therapy initiation.
Madhav Dasari
21 November, 2025Hey folks, just wanted to add that staying on top of the blood work schedule can really save you from unexpected dose reductions. I’ve seen patients who missed the bi‑weekly labs end up with severe neutropenia that could have been caught early. Also, keeping a simple diary of symptoms like fatigue or palpitations helps the oncologist tweak the dose before things get out of hand. And don’t forget to avoid grapefruit-it can really spike ribociclib levels.
DHARMENDER BHATHAVAR
27 November, 2025Good point; the lab timing is crucial for dose adjustment.
Kevin Sheehan
4 December, 2025The discourse around ribociclib often glosses over the fact that its marginal PFS gain does not necessarily translate into overall survival advantage for every subgroup. While the trials report a median OS benefit, the confidence intervals are wide, suggesting statistical uncertainty. Moreover, the QT prolongation, albeit infrequent, raises legitimate concerns for patients with baseline cardiac comorbidities. From a health‑economics perspective, allocating resources to a drug with modest incremental benefit warrants rigorous cost‑benefit analysis. In sum, enthusiasm should be tempered with critical appraisal of both efficacy and safety data.
ashanti barrett
10 December, 2025I’ve been following several patients on ribociclib and what strikes me most is how the side‑effect profile lets many maintain a relatively normal daily routine. The neutropenia can be scary, but with proper monitoring it’s usually manageable. Patients often report feeling less fatigued compared to traditional chemotherapy, which contributes to a better overall mood. It’s also reassuring to see that the drug’s mechanism specifically targets hormone‑driven cells, reducing collateral damage.
Sunil Yathakula
17 December, 2025yeah i seen ppl say the same, the med is kinda chill on the body once you get used to it
Catherine Viola
23 December, 2025It is imperative to acknowledge that the purported efficacy of ribociclib may be partially obfuscated by selective reporting within the pivotal trials, wherein adverse cardiac events could be under‑represented due to stringent inclusion criteria. Furthermore, the entanglement of pharmaceutical sponsorship with clinical research raises concerns regarding the impartiality of outcome measures. In light of these considerations, a rigorous independent meta‑analysis is warranted before endorsing widespread adoption of this agent.
sravya rudraraju
30 December, 2025When contemplating the integration of ribociclib into an oncologic treatment algorithm, it is essential to adopt a holistic perspective that encompasses not only the raw efficacy metrics but also the nuanced patient‑centric variables that influence therapeutic success. The regimen’s 21‑days‑on/7‑days‑off schedule aligns favorably with patient adherence patterns, yet it simultaneously imposes the responsibility of diligent monitoring for hematologic and hepatic perturbations. The necessity of periodic electrocardiographic assessments, dictated by the drug’s intrinsic propensity to prolong the QT interval, adds an additional layer of logistical complexity, particularly in resource‑constrained settings. Moreover, the economic dimension cannot be neglected; the substantial monthly cost, despite mitigation through assistance programs, may generate significant out‑of‑pocket expenditures for certain demographics, thereby engendering disparities in access. Nonetheless, the clinical benefits, as demonstrated by the MONALEESA series, present a compelling argument for its inclusion, provided that clinicians engage in shared decision‑making that transparently addresses both the therapeutic advantages and the attendant risks. Ultimately, the goal remains to extend survival while preserving quality of life, a balance that ribociclib, when administered judiciously, appears poised to achieve.
Ben Bathgate
5 January, 2026Sounds like a textbook pitch; in real clinics, the paperwork and follow‑ups often drown the supposed benefits.
Nicole Boyle
11 January, 2026Ribociclib’s place in the CDK4/6 landscape is pretty solid, especially when you factor in the modest but consistent PFS gains across multiple trials. For patients who can tolerate the monitoring demands, it offers a viable alternative to chemo with a more tolerable side‑effect profile.