TMP-SMX Dosage Calculator
Select Clinical Indication
Recommended Dosage
WARNING: Renal impairment detected. Dose adjustment required.
Consider reducing dose based on creatinine clearance.
When you hear the word trimethoprim, you probably think of a simple urinary‑tract infection pill. In reality, doctors often pair it with another drug to tackle tougher bugs and broaden coverage. This article breaks down why the combo works, where it shines, and what pitfalls you need to watch out for.
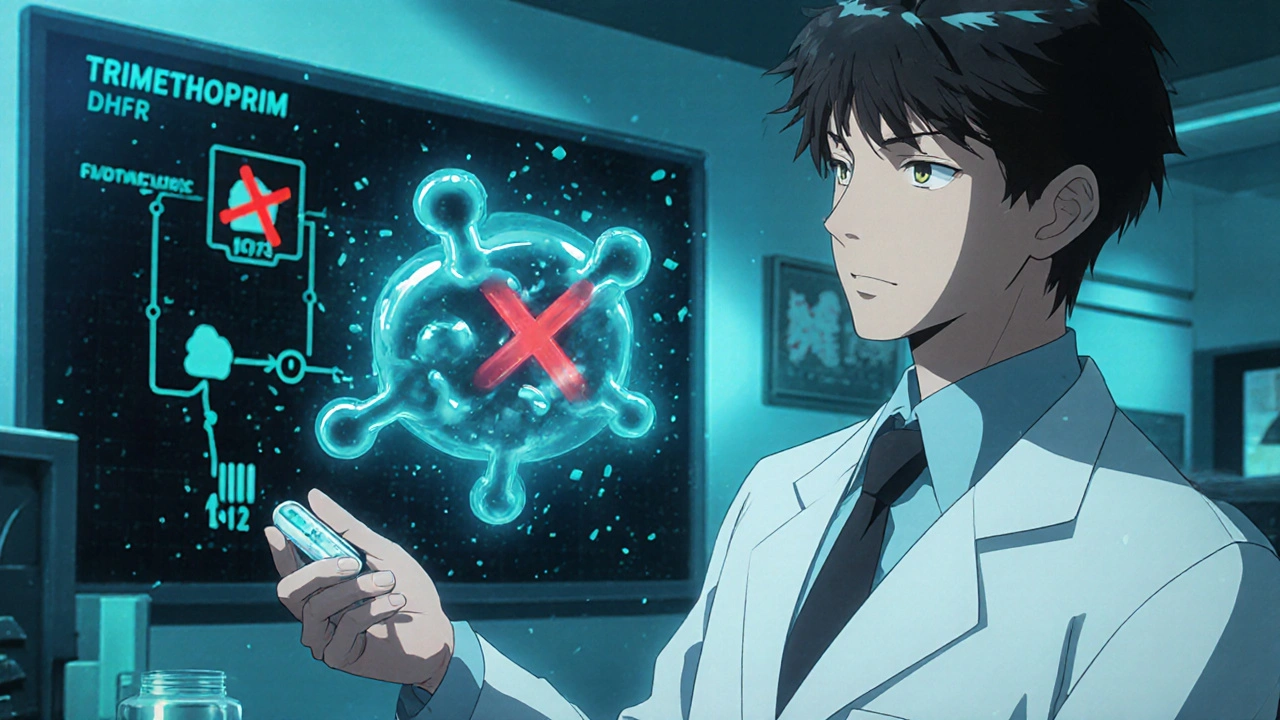
How Trimethoprim Works on a Molecular Level
Trimethoprim is a selective dihydrofolate reductase (DHFR) inhibitor that blocks bacterial folate synthesis, essential for DNA replication. By starving the microbe of tetrahydrofolic acid, it halts cell division without harming human cells, which obtain folate from the diet.
The drug is orally bioavailable, reaches high concentrations in urine, and has a half‑life of about 8‑10 hours, making it convenient for once‑ or twice‑daily dosing.
Why Combine? The Core Benefits of Combination Therapy
Monotherapy works for uncomplicated infections, but resistance can creep in fast. Pairing Sulfamethoxazole is a sulfonamide that inhibits dihydropteroate synthase, another step in the bacterial folate pathway creates a two‑pronged attack. The result is a synergistic block of folate production, dramatically reducing the chance that a single mutation will bypass both drugs.
- Broader antimicrobial spectrum - covers many Gram‑positive and Gram‑negative organisms.
- Lower emergence of resistance - the bacteria need two simultaneous mutations.
- Enhanced clinical efficacy - higher cure rates in pneumonia, skin infections, and opportunistic diseases.
Because of these advantages, the combination is marketed as Co‑trimoxazole (also called TMP‑SMX), a staple in many treatment guidelines.
Common Combination Partners and What They Add
While sulfamethoxazole is the classic partner, clinicians sometimes add other agents for special cases. Below are the most frequent pairings:
- Sulfamethoxazole - extends coverage to Staphylococcus aureus, Haemophilus influenzae, and many urinary pathogens.
- Metronidazole - used in anaerobic abdominal infections when the clinician wants to keep the DHFR blockade but needs extra anaerobic kill.
- Azithromycin - occasionally added for atypical pneumonia, though evidence is mixed.
In most everyday practice, though, the TMP‑SMX duo does the heavy lifting.

Clinical Situations Where the Combo Shines
Guidelines point to several high‑yield indications:
| Condition | Why TMP‑SMX fits | Typical dosage |
|---|---|---|
| Urinary tract infection (UTI) | High urinary concentrations, good activity against E. coli | 160 mg/800 mg PO BID for 3‑5 days |
| Pneumocystis jirovecii pneumonia (PJP) | Effective prophylaxis and treatment in immunocompromised patients | 800 mg/1600 mg PO QID for treatment; 1 tablet daily for prophylaxis |
| Travelers' diarrhea (bacterial) | Broad spectrum, covers Shigella and Salmonella | 160 mg/800 mg PO BID for 3 days |
| Skin and soft‑tissue infections | Good MRSA coverage when combined with other agents | As above, often with adjunctive clindamycin |
These scenarios illustrate why the combo is a workhorse in both community and hospital settings.
Challenges and Pitfalls to Keep an Eye On
Even a strong duo has drawbacks. Here are the most common red flags:
- Antimicrobial resistance - overuse in outpatient settings has driven resistance in E. coli and Staphylococcus species.
- Drug interaction - sulfonamides can amplify the effects of warfarin, leading to higher INR.
- Renal impairment - both drugs are cleared renally; dose reduction is needed when CrCl < 30 mL/min.
- Side‑effects such as rash, Stevens‑Johnson syndrome, and bone‑marrow suppression, especially in patients with folate deficiency.
- Hyperkalemia in patients on ACE inhibitors or potassium‑sparing diuretics.
Knowing these issues up front lets you weigh the risk‑benefit ratio and avoid surprises.
Practical Tips for Safe Prescribing
- Check renal function before starting; adjust to 80 mg/400 mg BID if CrCl < 30 mL/min.
- Ask about concomitant warfarin, methotrexate, or oral contraceptives - they may need dose tweaks or monitoring.
- Consider folic acid supplementation (1 mg daily) in patients at risk for bone‑marrow toxicity.
- Educate patients to report rash or severe nausea within the first 48 hours.
- For prophylaxis of PJP in HIV patients, stick to the low‑dose regimen (1 tablet daily) to limit toxicity.
Following these checkpoints can keep the therapy effective while minimizing adverse events.
Key Takeaways
- Combining Trimethoprim with Sulfamethoxazole creates a synergistic block of bacterial folate synthesis, lowering resistance risk.
- Core indications include uncomplicated UTIs, PJP prophylaxis/treatment, travelers' diarrhea, and certain skin infections.
- Watch for renal impairment, drug interactions (especially warfarin), and hypersensitivity reactions.
- Adjust dosing based on kidney function and supplement folate when needed.
- Reserve the combo for situations where its broad spectrum and proven efficacy outweigh the higher side‑effect profile.
Frequently Asked Questions
Can I use Trimethoprim alone for a simple UTI?
Yes, for uncomplicated infections caused by susceptible E. coli, trimethoprim 100 mg BID works well. However, local resistance rates above 20 % may push clinicians toward the combination.
Why does TMP‑SMX cause a rash more often than trimethoprim alone?
The sulfonamide component can trigger immune‑mediated skin reactions, especially in patients with a history of sulfa allergy. The risk is roughly 3‑5 % in the general population.
Is TMP‑SMX safe during pregnancy?
It’s Category D in the first trimester because sulfonamides can cause kernicterus in the newborn. In later trimesters, many clinicians still avoid it unless benefits clearly outweigh risks.
How do I monitor for bone‑marrow suppression?
Baseline CBC before starting therapy, then repeat weekly for high‑risk patients (e.g., those on methotrexate or with HIV).
What alternatives exist if a patient is allergic to sulfonamides?
Nitrofurantoin, fosfomycin, or a fluoroquinolone (if no contraindications) can replace TMP‑SMX for UTIs. For PJP, atovaquone or pentamidine are options.

11 Comments
Marrisa Moccasin
22 October, 2025Look, the pharma giants don’t want you to read about the hidden dangers of TMP‑SMX!!! They’re secretly pushing a cocktail of chemicals that could be used to control the population, and you’re just supposed to trust a bland “clinical tip”!!! Read the fine print, check the supply chain, and never assume a “standard dosage” is safe without a background check!!!
Oliver Johnson
24 October, 2025Patriotic America stands tall – we don’t bow to secret labs! Our doctors are the finest, and if they say TMP‑SMX works, it’s because they chose it, not because some shadowy cartel forced them. The truth is simple: we trust our own, not foreign conspiracies.
Taylor Haven
26 October, 2025The discussion surrounding trimethoprim‑sulfamethoxazole is not merely a pharmacological footnote, it is a moral indictment of a healthcare system that routinely sacrifices patient safety for profit. When the article extols the benefits of broad‑spectrum coverage, it conveniently glosses over the insidious ways in which pharmaceutical lobbying shapes guidelines to favor high‑margin drugs. One must recognize that the very notion of a “combination therapy” is a veil, obscuring the fact that each additional molecule introduces a new vector for adverse reactions, a fact that is systematically downplayed. Furthermore, the alleged synergy between trimethoprim and sulfamethoxazole, while scientifically plausible, is repeatedly weaponized to justify higher dosing without adequate monitoring of renal function. Patients with diminished creatinine clearance are told to “adjust the dose,” yet the onus of calculation is placed on the individual rather than enforced by prescribers, a clear abdication of responsibility. The article’s brief nod to drug interactions with warfarin, for instance, fails to mention the cascade of events that can culminate in life‑threatening hemorrhage, a risk that is far too often dismissed as an “exception.” Equally concerning is the casual reference to folic acid supplementation, which may mask the underlying issue that sulfonamides themselves can precipitate folate deficiency, turning a simple prophylactic measure into a band‑aid. In the broader epidemiological context, the rising prevalence of sulfa‑resistant E. coli strains is a direct consequence of overprescribing TMP‑SMX, a phenomenon that public health officials are reluctant to own. It is a testament to systemic failure when a drug class designed to combat infection becomes a driver of antimicrobial resistance, yet the narrative is framed as a triumph of modern medicine. The ethical lapse extends to the omission of alternative therapies, such as nitrofurantoin or fosfomycin, which could serve as viable options without the burden of sulfa‑related toxicity. By presenting the combination as a “workhorse,” the article tacitly endorses a one‑size‑fits‑all approach that erodes the principle of individualized care. Healthcare providers are urged to consider patient‑specific factors, yet the guidelines are written in a language that speaks only to the pharmaceutical agenda. The irony is that a drug that interferes with folate metabolism-a pathway essential to human cell division-is promoted without a robust discussion of its potential impact on rapidly dividing bone‑marrow cells. All this is compounded by the fact that the article fails to address the psychosocial implications of adverse skin reactions, which can leave patients with scarring and a lasting distrust of medical interventions. Thus, the narrative that glorifies TMP‑SMX must be re‑examined through the lens of patient safety, ethical stewardship, and transparent risk communication. Only then can we claim that we are truly serving the public good rather than perpetuating a cycle of dependency on a drug cocktail designed for profit.
Vandermolen Willis
27 October, 2025Hey everyone! 🙌 Great points about the risks – I’ve seen a few patients develop mild rashes, so I always double‑check kidney labs before continuing. Staying on top of those labs makes the combo feel safer. 😊
Mary Keenan
29 October, 2025Don’t overcomplicate it – TMP‑SMX works when used correctly.
Kelly Brammer
31 October, 2025The article accurately notes the necessity of renal dose adjustment, yet it could further emphasize that creatinine clearance must be calculated using the Cockcroft‑Gault formula for precise dosing, thereby minimizing the risk of accumulation and adverse events.
Ben Collins
1 November, 2025Sure, because nothing says “wise prescription” like throwing in a sulfonamide and hoping the patient’s immune system can handle the fireworks. 🙃
Denver Bright
3 November, 2025From a worldwide standpoint, the reliance on TMP‑SMX reflects limited access to newer antibiotics, which is both a challenge and an opportunity for stewardship.
Kelli Benedik
5 November, 2025OMG, the drama of a rash that looks like a bad tattoo 😱! One moment you’re feeling fine, the next you’re battling skin that screams “stop!” – it’s a rollercoaster of emotions when TMP‑SMX decides to throw a surprise party on your epidermis! 🎢💥
cariletta jones
7 November, 2025Remember to check kidney function and keep an eye on drug interactions – safety first!
Kevin Hylant
8 November, 2025Is there a quick way to know if a patient is at risk for hyper‑kalemia when they’re also on an ACE inhibitor?